ReValve Med, Inc
Overview
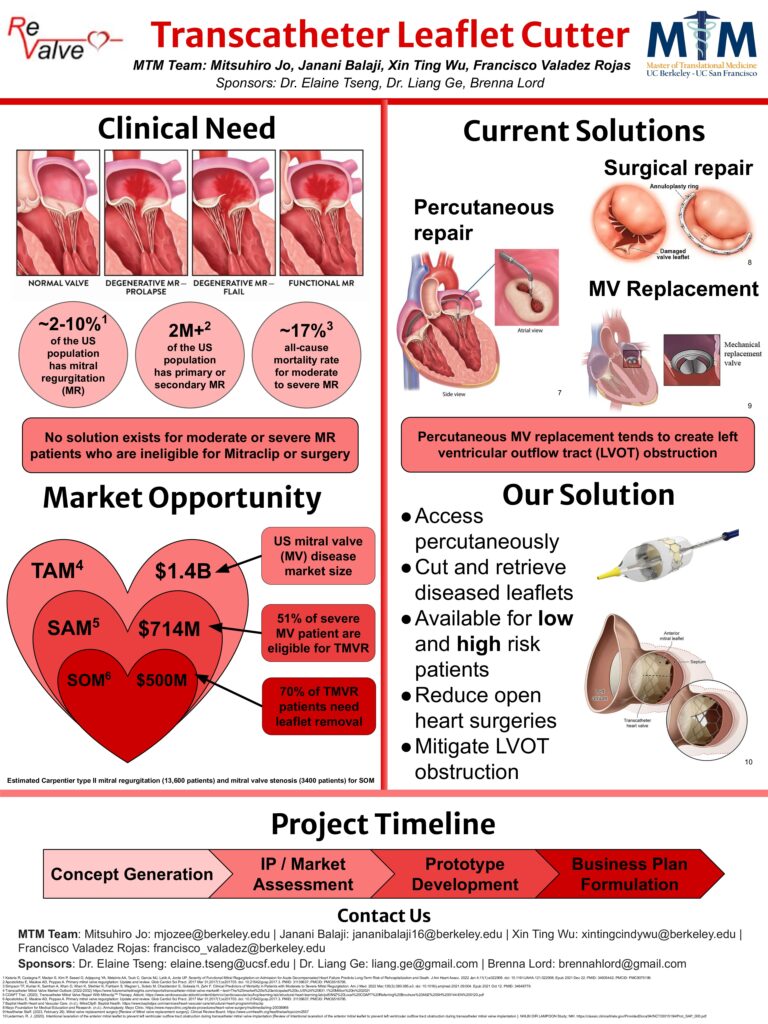
ReValve is developing a solution for Transcatheter Aortic Valve Replacement (TAVR) patients who will outlive the limited durability of current TAVR options.
The Issue
TAVR durability is limited in bioprosthetic devices to around 8 years. Patients under 75 years old who undergo TAVR procedures only have two options after the outlived TAVR fails: open heart surgery or valve-in-valve implantation. Each has risks and may not be possible for some patients.
Approach
ReValve is developing two devices which work as a system: one focused on removing and retrieving degenerated TAVR, bioprosthetic, or native valves, and the other focused on stabilizing patient hemodynamics during implantation of a new TAVR.
MTM Student Engagement
Our capstone team refined the optimal prototypes among the IP already submitted for patent application. Additionally, we created the user needs experience, market assessment, and business plan for the devices, as well as examined the IP, FTO analysis, and planned the regulatory strategy.